Quantum Mechanics & Electron Configuration
580 likes | 878 Views
Quantum Mechanics & Electron Configuration. Chapter 5: Electrons in Atoms. Part 1: Models of the Atom. 1897: Thompson Model (Plum Pudding) 1911: Rutherford Model – Small, dense, + charged nucleus Electrons orbit around 1913: Bohr Model 1926: Quantum Mechanical Model –

Quantum Mechanics & Electron Configuration
E N D
Presentation Transcript
Quantum Mechanics & Electron Configuration Chapter 5: Electrons in Atoms
Part 1: Models of the Atom 1897: Thompson Model (Plum Pudding) 1911: Rutherford Model – Small, dense, + charged nucleus Electrons orbit around 1913: Bohr Model 1926: Quantum Mechanical Model – Erwin Schrodinger & his math equations
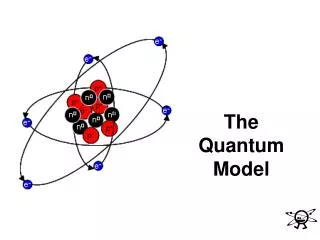
Bohr Model (aka the versions you’ve learned before) • Electrons move around the nucleus in fixed spherical orbits with fixed energies • Fixed energies = orbits / energy levels • Aka rungs of a ladder • Electrons can go to a higher or lower energy level • Either gain or lose energy to move levels • Electrons CANNOT be between levels
Atomic Emission Spectra ** When atoms absorb energy (i.e. electric current), they move to a higher energy level … … these electrons emit light when they return back to a lower energy level • Emission spectra is unique for each element • The light emitted consists of only a mixture to specific frequencies… • If you pass the light through a slit and then a prism, you can separate the resulting light into its frequencies (aka colors) Barium
Light • Has properties of both: a Particle ( ____________) a Wave Light Waves: Amplitude: crest of the wave (height from 0) Wavelength: distance between crests (λ) Frequency: # of waves per unit time (ν) Units: Hertz (Hz) aka s-1
Math Time!!! c = λν C = speed of light (constant) = 2.998 x 108 m/s λ = Wavelength (m) ν = Frequency (Hz or s-1)
More Math… • The energy (E) of a photon is directly proportional to its frequency. Higher freq = More Energy Lower Freq = Less Energy E = h x v E = energy (joules – J) H = Plank’s constant = 6.626E-34 J/s v = Frequency (Hz or s-1)
Example: What is the energy of a quantum of light with a frequency of 7.39 x 1014 Hz?
Think about this… • E = h x v • c = λν What would you do if you were asked to solve for the frequency of light if you are given a wavelength of 700nm? What would you do if you were asked to find the energy of light if you are given a wavelength of 480nm?
Emission Spectra Lab Look at the gas tubes and follow directions provided.
Continuous Spectrum v. Line Spectrum • What did you observe in the Emission Lab?
Light has Wave-Particle Duality (& so do electrons) • Particle & Wave-like Nature • Depends on experiment / what we try to observe • Throws a wrench in Bohr Model… • New method of describing the motion of subatomic particles = foundation of quantum mechanics = movement/organization of subatomic particles
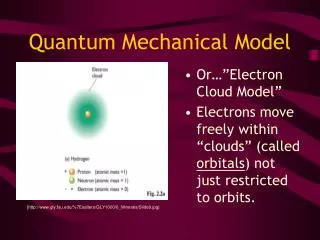
The Quantum Mechanical Model • This is what we use today • Describes: LOCATION & ENERGY of electrons • Electrons do not have a direct orbit around nucleus • Based on probability • Electron clouds • Electrons do have energy levels
Hog Hilton Sample Problem • Book 15 hogs into their rooms • 6th floor ____ ____ ____ _____ _____ • 6th floor ______ • 5th floor ______ ______ ______ • 4th floor ______ • 3rd floor ______ ______ ______ • 2nd floor ______ • 1st floor ______
Hog Hilton Sample ProblemPlace 15 electrons into their spaces • 3d_____ _____ _____ _____ ____ • 4s _____ • 3p ______ ______ ______ • 3s ______ • 2p ______ ______ ______ • 2s ______ • 1s ______
But…all of these electrons are not organized into hotel rooms, but ATOMIC ORBITALS
So, what exactly is an ATOMIC ORBITAL? Atomic Orbital = region of space in which there is a high probability of finding an electron • They come in different SHAPES, SIZES & ENERGY LEVELS!! • These are described by Quantum Numbers…
Part 2 Quantum Numbers Get ready…here we go…
Quantum Numbers Used to describe the location of electrons Electrons in an atom CANNOT have the same quantum numbers Unique for each electron Like an address
Principle Quantum Number (think…Energy Level) • n • Allowable values = 1, 2, 3 … n (positive, integer values) • Describes energy level • Position of the electron w/ respect to nucleus • As n increases = further from nucleus
Angular Momentum Quantum Number(Azimuthal Quantum Number)(think…energy sublevel)Pay attention…this is where it starts to get complicated • l • Allowed values: 0, 1, 2, … (n-1) • Describes the sublevel • SHAPE of the orbital • SHAPES: • l = 0 = s orbital = spherical cloud • l = 1 = p orbital = dumbbell cloud • l = 2 = d orbital = clover cloud • l = 3 = f orbital = … too complicated
Example • If I had a principal quantum number of 2, what are my possible angular momentum quantum numbers? n = 2 l =
Magnetic Quantum Number (ml) • Determines spatial orientation (x, y, z, plane) • Possible Values: - l to + l • Examples: if it is a d orbital d orbital: l = ml =
Example: p-orbital n = 2 l = ml = This means, there are _______ p-orbitals and that they are in three directions (x, y, z axes):
What orbital corresponds to :n = 2l = 1ml = 0 Energy level = Sublevel = _____ - orbital Orientation: Orbital:
Number of orbitals within an energy level: n2 Examples: How many orbitals are in energy level 2? n = l = ml = Orbitals = • Each orbital holds 2 electrons:So, how many electrons can energy level 2 hold? # Electrons = 2n2
Spin Quantum Number • ms • Describes the direction of the electrons spin within an orbital (remember, each orbital only holds 2 electrons) • Possible Values: ½ or -½ (spin up, spin down) • Think back to hogs…
Ahhh…it’s too much information…HELP!!! • Solution: STUDY and PRACTICE!!!
Examples • n = 3 (what are the possible quantum numbers?) • What orbital corresponds to n = 4 & l = 2?
What orbital corresponds to n = 4 , l = 1, ml = -1 Energy Level = Sublevel = Orbital orientation = Orbital =
PART 3 Rules of Electron Configuration
Aufbau Principle • Electrons enter orbitals of lowest energy first • Orbitals within a sublevel have equal energy (3px, 3py, 3pz) • Exceptions: Cr , Cu • Which hog rules is this?
Pauli Exclusion Principle • An atomic orbital may only hold two electrons • Electrons must have opposite spin • Clockwise or counterclockwise spin • Denoted with arrows • Prevents two electrons from having same quantum numbers • Which hog rule is this?
Hund’s Rule • Every orbital of the same energy is singly occupied before any orbital is doubly occupied • Electrons have the same spin • Second electrons added have opposite spins • Which hog rule is this?
PART 4 Writing Electron Configurations
Electron Configuration Diagonal Rule • Starting with the top arrow, follow the arrows one by one in the direction they point, listing the sublevels as you pass through them. • Stop when you get to the sublevel you need.
Electron Orbital Diagram 3d ___ ___ ___ ___ ___ 4s ___ 3p ___ ___ ___ 3s ___ 2p ___ ___ ___ 2s ___ 1s ___
Example: Fill Orbitals w/ 7 electrons 3d ___ ___ ___ ___ ___ 4s ___ 3p ___ ___ ___ 3s ___ 2p ___ ___ ___ 2s ___ 1s ___
Review: • How many electrons fill an s orbital? • How many electrons fill a p orbital ?(remember subshells…) • How many electrons fill a d orbital? • How many electrons fill an f orbital?
Example: Cl 3d ___ ___ ___ ___ ___ 4s ___ 3p ___ ___ ___ 3s ___ 2p ___ ___ ___ 2s ___ 1s ___ Give the final E.C:
With a partner:Examples: Give the E.C • H • He • Li • Be • B • C • N • F
No more…Make it stop!@!!!! • Write the electron configuration for Barium: • Ahhhhhhhhhh!!! Too many electrons!! • But wait…there’s a shortcut… • Noble gas / shorthand configuration: • Find the nearest noble gas that came before the element you are interested in • Write the symbol of that noble gas in [brackets] • Write the configuration as normal from there…