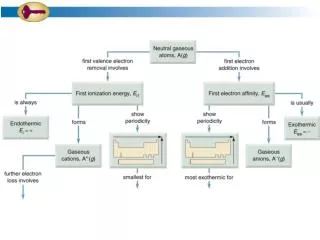
Kj mol - PowerPoint PPT Presentation
View Kj mol PowerPoint (PPT) presentations online in SlideServe. SlideServe has a very huge collection of Kj mol PowerPoint presentations. You can view or download Kj mol presentations for your school assignment or business presentation. Browse for the presentations on every topic that you want.