In industries like pharmaceuticals, life sciences, manufacturing, aerospace, and healthcare, equipment calibration plays a vital role in ensuring accuracy, compliance, and product quality. An uncalibrated or poorly tracked instrument can lead to costly errors, compliance violations, or even product recalls.
To overcome these challenges, organizations are turning to digital calibration management and tracking software systems like Qualityze. The Need for Calibration Management Calibration is the process of verifying that equipment and instruments are working within the acceptable range of accuracy. Without a robust system in place, companies often struggle with: Missed calibration schedules Manual errors in record-keeping Difficulty retrieving calibration certificates Incomplete audit trails Noncompliance with standards such as ISO 9001, FDA 21 CFR Part 11, and GMP
A cloud-based calibration management system ensures every instrument is calibrated on time and all records are readily available for audits. What Is Qualityze Calibration Management and Tracking Software?
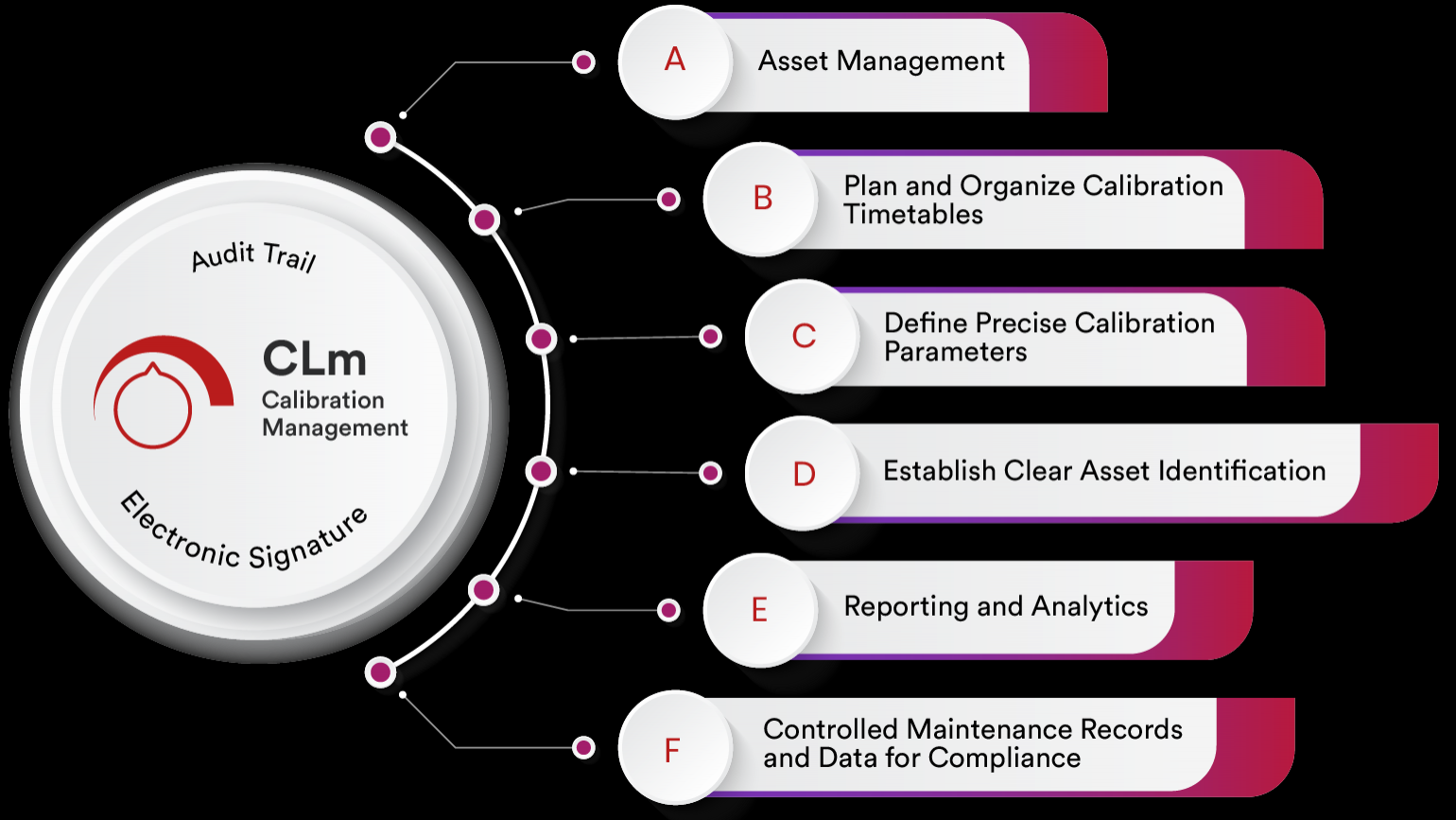
Qualityze Calibration Management Software is a Salesforce-powered, cloud-native solution that helps organizations plan, schedule, execute, and track calibration activities seamlessly. It provides complete visibility into equipment history, calibration status, and future schedules. By digitizing the entire calibration lifecycle, Qualityze enables organizations to maintain accuracy, traceability, and compliance effortlessly. Key Features of Qualityze Calibration Management & Tracking System
✅ Automated Calibration Scheduling – Never miss calibration dates with alerts and notifications. ✅ Centralized Equipment Records – Maintain a digital repository of all assets, calibration history, and certificates. ✅ Criteria Library – Configure measurement tolerances and repeatability/reproducibility (R&R) studies. ✅ Audit-Ready Documentation – Generate and store calibration certificates with secure audit trails. ✅ Out-of-Tolerance Handling – Detect deviations quickly and trigger nonconformance or CAPA processes. ✅ Emergency Calibration Support – Handle unscheduled calibrations when issues arise. ✅ Integration with EQMS Modules – Seamlessly connects with CAPA, Audit, Training, and Nonconformance management for closed-loop quality.
Benefits of Implementing Qualityze Calibration Management Ensure Regulatory Compliance – Meet global standards like ISO, GMP, and FDA 21 CFR Part 11. Improve Equipment Reliability – Prevent downtime and extend asset life. Save Time & Costs – Reduce manual tracking and paperwork. Enhance Data Accuracy – Secure digital records and e-signatures improve data integrity.
Be Audit-Ready Anytime – Quickly retrieve calibration records and certificates. Boost Productivity – Free up resources from manual tasks to focus on quality improvement. Why Choose Qualityze?
Unlike traditional calibration systems, Qualityze is scalable, cloud-based, and user-friendly. Built on the Salesforce platform, it ensures: Enterprise-grade security and accessibility Customizable workflows to fit industry-specific needs Scalability for organizations of all sizes Seamless collaboration across teams and sites Final Thoughts Calibration is not just a compliance requirement—it is the backbone of accuracy, safety, and trust in products and services. With Qualityze Calibration Management and Tracking Software, organizations can move away from manual, error-prone processes and embrace a digital, efficient, and compliant system. By investing in Qualityze, businesses ensure equipment accuracy, audit readiness, and long-term customer confidence.