Understanding pH and pOH: Calculations and Relationships of Ion Concentrations
80 likes | 352 Views
This article delves into the concepts of pH and pOH, fundamental parameters in chemistry representing hydrogen and hydroxide ion concentrations, respectively. Learn how to calculate pH using the formula pH = -log[H+] and the inverse calculation of [H+] from pH. Observe patterns in ion concentrations across various pH levels and explore the relationship between pH and pOH. Understand the pOH calculation (pOH = -log[OH-]) and the important equilibrium relationship pH + pOH = 14, crucial for grasping aqueous solution chemistry.

Understanding pH and pOH: Calculations and Relationships of Ion Concentrations
E N D
Presentation Transcript
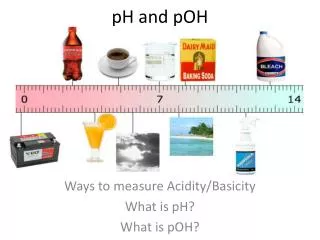
pH pH: “potential of hydrogen” - A way of expressing the hydrogen ion concentration pH = - log [H+] [H+] = the hydrogen ion concentration
Calculating pH Calculate the pH values using the equation pH = - log [H+]. Do you notice a pattern? 3.0 5.0 7.0 9.0 12.0
How do we calculate [H+] if we know the pH? pH = - log [H+] [H+] = the hydrogen ion concentration [H+] = 10-pH
Calculating [H+] Calculate the [H+] values using the equation [H+] = 10-pH. Do you notice a pattern? 1 x 10-3 M 1 x 10-5 M 1 x 10-7 M 1 x 10-9 M 1 x 10-12 M
Calculating pH [H+] > 1 x 10-7 [H+] = 1 x 10-7 [H+] < 1 x 10-7
The pH Scale - As pH increases, [H+] decreases - As pH decreases, [H+] increases
pOH pOH: “potential of hydroxide” pOH = - log [OH-] Just like pH: - pOH is a convenient way of expressing the hydroxide ion concentration [OH-] = the hydroxide ion concentration Just like [H+]: [OH-] = 10-pOH pH + pOH = 14