Unraveling Atomic Structure: Journey through Electron Orbits
150 likes | 252 Views
Explore the evolution of atomic theory, from Bohr's model to modern wave models by Schrodinger, Heisenberg's uncertainty principle, and the structure of subatomic particles like protons, neutrons, and electrons.

Unraveling Atomic Structure: Journey through Electron Orbits
E N D
Presentation Transcript
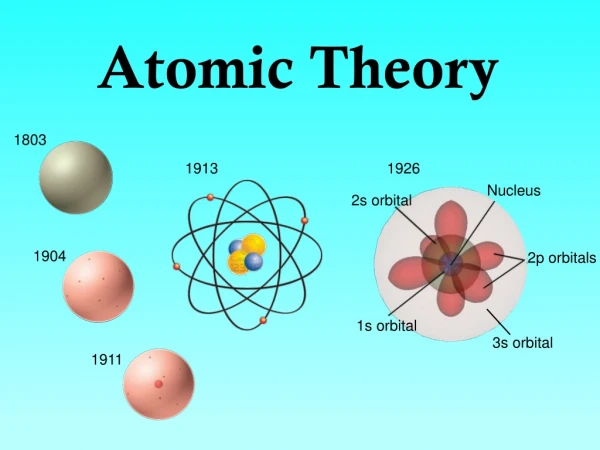
Bohr Model (1913) • electrons move in definite orbits around the nucleus • these orbits or energy levels are located at certain distances from the nucleus
Bohr’s Model nucleus Electrons
Wave Model (present day) • based on complex math equations • orbits are more complex than originally thought • de Broglie stated that electrons (particles) have wave properties, and he viewed these as standing waves, like those produce when a guitar string is plucked (classical physics.) • Schrodinger assumed that the electron in Hydrogen behaves as a standing wave.
Wave Model (continued) • When Schrodinger’s equation is analyzed, many solutions are found. • Each solution represents an atomic orbital. • An atomic orbital is the most probable location for finding an electron.
What is an orbital? • It is not a Bohr orbit (not moving in a circular path.) • How is the electron moving? • We don’t know! • There is a fundamental limitation to just how precisely we can know both the position and momentum of a particle at a given time
Heisenberg Uncertainty Principle • The more accurately we know the particle’s position, the less accurately we can know it momentum and vice versa. • We can’t know the exact motion of the electron around the nucleus. • The area that an electron orbits is called an “electron cloud”
Subatomic Particles • subatomic-lower (or smaller) than an atom • Protons-positive particles • Neutrons-neutral particles • Electrons-negative particles
Location of particles • Protons and neutrons are in the nucleus (core) of the atom. • electrons are buzzing around the nucleus in the electron cloud or shell. • The nucleus makes up 99.99% of the mass of the atom. • You compared to pocket lint • The nucleus is 1/100,000 of the volume of an atom • a marble compared to a football stadium
Mass of particles • Since subatomic particles are so small they cannot be measured in grams • instead they are measured in atomic mass units or amu • 1 amu = 1.61x10-24 g • remember 1 g is about the mass of a paper clip