Cells & their Membranes
610 likes | 631 Views
Explore the fascinating world of cell membranes, diffusion, active transport, and osmosis. Learn how molecules move, membrane structures, and the importance of concentration gradients.

Cells & their Membranes
E N D
Presentation Transcript
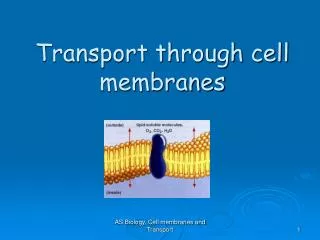
Diffusion Movement of molecules from high concentration to low concentration
Diffusion • This occursnaturally so does NOT require energy • Examples: opening a perfume bottle in closed room; drop of dye in water • NOTE: molecules are constantly moving, even in a solid
Diffusion • The difference in concentration is a concentration gradient things naturally move down their gradients
Cell Membranes • Impermeable • Completely permeable • Selectively permeable (semipermeable)
Cell Membranes • Which molecules are permeable (enter by diffusion)? • Fat-soluble compounds • nonpolar! • Water • Interacts with polar region of phospholipid and is a small molecule • Na+, K+, Cl- ions can diffuse but slowly • Small monomers (i.e., glucose, amino acids)
Cell Membranes • Impermeable to: • Larger molecules: disaccharides, sucrose
Membrane structure • All cell membranes seem to be phospholipid bilayers! • Cholesterol helps stablilize membranes (gives it some rigidity) • Protein molecules vary in size
Fluid Mosaic Model • Why fluid? • Why mosaic?
Facilitated diffusion • Carries materials across cell membrane according to concentration gradient (therefore, without energy-- ATP) • Carrier proteins = permeases
Active Transport • Usually carries molecules AGAINST their concentration gradient (therefore, requires energy-- ATP!)
acts as a pore for molecules Active TransportGramicidin: an ion-channel
Active TransportSodium-Potassium Pump • The pump is an enzyme (a Na+ & K+ dependent ATPase) • Important to normal functioning of nerve and muscle cells • builds electro-chemical energy to release impulse think about a water dam or a crowded elevator
Active TransportSodium-Potassium Pump • The pump is an enzyme (a Na+ & K+ dependent ATPase) • Important to normal functioning of nerve and muscle cells • builds electro-chemical energy to release impulse think about a water dam or a crowded elevator • Na-K pump also allows glucose & amino acids to be carried into the cell
Movement of large particles • Endocytosis: “swallowing” large pieces • phagocytosis: take in pieces • pinocytosis: take in liquids or macromolecules
Movement of large particles • Exocytosis: materials released as digestive enzymes in membrane-lined sacs -- vesicles
Osmosis • Diffusion of water through a selectively permeable membrane • Concentration = [ ]
The solute (large circles) is more concentrated on the right side, which pulls the water molecules toward that side. The large circles would move to the left to spread out evenly, BUT the membrane won't let those pass. Osmosis
Osmosis: Terms to know… • Hydrostatic pressure: the pressure exerted at any given point of a non-moving (static) fluid. push of water
Osmosis: Terms to know… • osmotic pressure (osmotic “pull”): the pressure applied by a solution to prevent inward flow of water across a semipermeable membrane • The greater the pressure of a solution, the more water is pulled into it
Osmosis: Terms to know… • osmotic potential: negative osmotic pressure; a solution’s tendency to gain water when separate from pure water • = “water concentration” = [H2O]
HYPOTONIC HYPERTONIC • Low [solute] • Low osmotic pressure • high osmotic potential • Med [solute] • Med osmotic pressure • Med osmotic potential
HYPERTONIC HYPOTONIC • High [solute] • High osmotic pressure • Low osmotic potential • Low [solute] • Low osmotic pressure • -High osmotic potential
Osmosis • Water will move from an area of lower osmotic pressure (pull) to an area of higher osmotic pressure (pull)
A Pure water HYPERTONIC HYPOTONIC • low [solute] • low osmotic pressure • high osmotic potential • zero [solute] • zero osmotic pressure • zero osmotic potential
B Pure water HYPERTONIC HYPOTONIC • med [solute] • med osmotic pressure • med osmotic potential • zero [solute] • zero osmotic pressure • zero osmotic potential
C Pure water HYPERTONIC HYPOTONIC • high [solute] • high osmotic pressure • low osmotic potential • zero [solute] • zero osmotic pressure • zero osmotic potential
H2O moves C B Increasing osmotic pressure A 0 pure H2O Increasing [solute] a Decreasing osmotic potential b c H2O moves
Solutions • -tonic = solute • Hypotonic: more water than what it’s adjacent to; less solute • Hypertonic: less water; more solute • Isotonic: equal water & solute