Chapter 14 - Biogeochemical Cycling
510 likes | 703 Views
Chapter 14 - Biogeochemical Cycling. Objectives Be able to give an explanation of why biogeochemical cycles are important Be able to explain what the GAIA hypothesis is Be able to list three major biogeochemical changes between early and modern earth

Chapter 14 - Biogeochemical Cycling
E N D
Presentation Transcript
Chapter 14 - Biogeochemical Cycling • Objectives • Be able to give an explanation of why biogeochemical cycles are important • Be able to explain what the GAIA hypothesis is • Be able to list three major biogeochemical changes between early and modern earth • Be able to define the term reservoir and give an example of a small easily perturbed reservoir and a large stable reservoir • Be able to list the three major plant polymers • Be familiar with all parts of the carbon, nitrogen, and sulfur cycles • Be able to draw each cycle and describe the microbial activities associated with each leg of the cycles • Be able to give an example of a microbe associated with each leg of the cycle
Chemical composition of an E. coli cell
How has earth maintained conditions favorable for life? Compare atmospheres and temperatures on Earth, Venus, and Mars. Atmosphere and Temperatures found on Venus, Mars, and Earth Gas Venus Mars Earth no life Earth with life Carbon dioxide Nitrogen Oxygen Argon Methane Surface temperature 0C 96.5% 3.5% Trace 70 ppm 0 459 95% 2.7% 0.13% 1.6% 0 -53 98% 1.9% 0 0.1% 0 290 50 0.03% 79% 21% 1% 1.7ppm 13
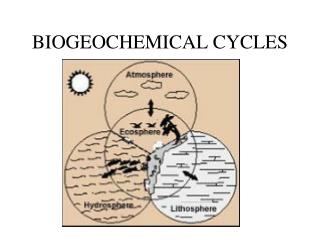
Biogeochemical activities are: unidirectional on a geologic time scale cyclical on a contemporary scale The concept of a reservoir To understand cycling of elements, the size and cycling activity level of the reservoirs of the element must be defined. atmospheric CO2 is a relatively small reservoir of carbon that is actively cycled. Such small, actively cycled reservoirs are most subject to perturbation.
What reactions drive biogeochemical cycling? Physical transformations dissolution precipitation volatilization fixation Chemical transformations biosynthesis biodegradation oxidoreductive-biotransformations Driving force for biogeochemical cycles is sunlight The ability to photosynthesize allows sunlight energy to be trapped and stored. This is not an efficient processalthough some environments are more productive than others.Only 10-15% of the energy trapped in each trophic level is passed on to the next level.
Net primary productivity of some natural and managed ecosystems
The Carbon Cycle The development of photosynthesis allowed microbes to tap into sunlight energy and provided a mechanism for the first carbon cycle. At the same time the carbon cycle evolved, the nitrogen cycle emerged because nitrogen was limiting for microbial growth. Although N2 was present, it was not in a usable form for microbes.
The carbon cycle is a good example of one that is undergoing a major perturbation due to human activity. Human activity has had a large impact on the atmospheric CO2 reservoir beginning with industrialization. As a result, the level of CO2 in the atmosphere has increased 28% in the past 150 years. Carbon sourcemetric tons carbon/yr Release by fossil-fuel combustion 7 x 109 Land clearing 3 x 109 Forest harvest and decay 6 x 109 Forest regrowth -4 x 109 Net uptake by oceans -3 x 109 Annual flux 9 x 109
Natural and anthropogenic CO2 sources and sinks • Natural sources of CO2 • respiration • ocean degassing • terrestrial degassing • wildfires • Anthropogenic sources of CO2 • fossil fuel combustion • cement production • land use changes • Natural sinks for CO2 • terrestrial • uptake by plants • uptake by soils • oceanic • partitioning • biomass production • Anthropogenic sinks for CO2 • chemical production • biological materials
CO2 is not the only problem! Global Atmospheric Concentrations of Selected Greenhouse Gases CH4 is 22 times stronger as a greenhouse gas than CO2
Carbon cycling on the habitat scale The term reservoir can be used on a global scale or on a smaller scale such as a habitat. How does carbon cycle within a habitat? • Macro vs. microorganisms • simple vs. simple to complex substrates • aerobic vs. aerobic/anaerobic redox conditions • What are the major carbon inputs into the environment? • plant materials (through photosynthesis) • cellulose 15 – 60% • hemicellulose 10-30% • lignin 5- 30% • protein/nucleic acids 2-15% • fungal cell walls/arthropods • chitin
Cellulose Cellulose degradation begins outside the cell with a set of three exoenzymes: β-1,4- endoglucanse β-1,4- exoglucanase β-1,4- glucosidase
Hemicellulose Chitin
For the more complex polymers such as lignin a variety of oxidizing enzymes are used. A specific example is the combination of lignin peroxidase and oxidase which produce H2O2 to aid in degradation of lignin. Lignin due to its complexity is generally degraded much more slowly than cellulose or hemicellulose.
The most complex organic polymer found in the environment is humus. Formation of humus is a two-stage process that involves the formation of reactive monomers during the degradation of organic matter, followed by the spontaneous polymerization of some of these monomers into the humus molecule.
Ultimately, these large polymers are degraded and produce new cell mass, CO2 (which returns to the atmosphere), and contribute to the formation of a stable organic matter fraction, humus. Humus turns over slowly, at a rate of 3 to 5% per year. In addition to mineralization to CO2, a number of small carbon molecules are formed largely as a result of anaerobic activities and in some instances as a result of anthropogenic activity. These include: Methane generation The methanogens are a group of obligately anaerobic Archaea that can reduce CO2 to methane (use CO2 as a terminal electron acceptor) both chemoautotrophically or heterotrophically using small MW molecules such as methanol or acetate. 4H2 + CO2 CH4 + 2H2O G0 = -130.7 kJ Although much methane is microbially produced, there are other sources as well. What happens to the methane? This is of concern because methane is a greenhouse gas 22 times more effective than CO2 in trapping heat.
Anthropogenic 190 – 405 54 - 49% of total
CH4 + O2 CH3OH HCHO HCOOH CO2 + H2O Methane utilization In most environments, the methane produced is utilized by methanotrophic microbes as a source of carbon and energy. The first enzyme in the biodegradation pathway of methane is methane monooxygenase (MMO). This enzyme is of interest because it can aid in the degradation of highly chlorinated materials such as TCE (trichloroethylene). The oxidation of TCE does not provide energy for the microbe, it is simply a result of nonspecific catalysis by the MMO enzyme. This is also called cometabolism. MMO methanol formaldehyde formic acid
Carbon monoxide- a highly toxic molecule that is produced largely as a result of fossil fuel burning and photochemical oxidation of methane in the atmosphere. Despite the fact that this is a highly toxic molecule, some microbes can utilize is as a source of energy. CO2 CO CO2 CO CO2 CO In summary, there is huge variety in the types of carbon-containing molecules found in the environment. Similarly microbes have developed an equal variety in their metabolic approaches to deriving carbon and energy from these compounds.
The Nitrogen Cycle N is cycled between: NH4+ (-3 oxidation state) and NO3- (+5 oxidation state)
Nitrogen must be fixed before it can be incorporated into biomass. This process is called nitrogen fixation. Biological inputs of nitrogen from N2 fixation land - 135 million metric tons/yr (microbial) The enzyme that catalyzes nitrogen fixation is nitrogenase. marine - 40 million metric tons/yr (microbial) fertilizers - 30 million metric tons/yr (anthropogenic) Rates of Nitrogen Fixation 1-2 kg N/hec/yr 2- 25 kg/N/hec/yr
Examples of free-living bacteria: Azotobacter - aerobic - aerobic, likes acidic soils Beijerinckia Azospirillum - facultative Clostridia - anaerobic Free-living bacteria must also protect nitrogenase from O2 complex is membrane associated slime production high levels of respiration conformation change in nitrogenase when O2 is present
} assimilation and mineralization Summary for nitrogen fixation: energy intensive end-product is ammonia inhibited by ammonia occurs in aerobic and anaerobic environments nitrogenase is O2 sensitive Fate of ammonia (NH3) produced during nitrogen fixation plant uptake microbial uptake adsorption to colloids (adds to CEC) fixation within clay minerals incorporation into humus volatilization nitrification
Release of assimilated NH3 is called ammonification. This process can occur intracellularly or extracellularly proteases chitinases nucleases ureases Ammonia assimilation and ammonification NH3 is assimilated by cells into: proteins cell wall constituents nucleic acids
At high N concentrations At low N concentrations
Summary for ammonia assimilation and ammonification Assimilation and ammonification cycles ammonia between its organic and inorganic forms Assimilation predominates at C:N ratios > 20 Ammonification predominates at C:N ratios < 20 Fate of ammonia (NH3) produced during nitrogen fixation plant uptake microbial uptake adsorption to colloids (adds to CEC) fixation within clay minerals incorporation into humus volatilization nitrification
Nitrification - Chemoautotrophic aerobic process Nitrosomonas Nitrobacter NH4+ NO2- NO3- Nitrosomonas: 34 moles NH4+ to fix 1 mole CO2 Nitrobacter: 100 moles NH4+ to fix 1 mole CO2 Nitrification is important in areas that are high in ammonia (septic tanks, landfills, feedlots, dairy operations, overfertilization of crops). The nitrate formed is highly mobile (does not sorb to soil). As a result, nitrate contamination of groundwater is common. Nitrate contamination can result in methemoglobenemia (blue baby syndrome) and it has been suggested (not proven) that high nitrate consumption may be linked to stomach cancer. Summary for nitrification Nitrification is an chemoautotrophic, aerobic process Nitrification is sensitive to a variety of chemical inhibitors and is inhibited at low pH. (There are a variety of nitrification inhibitors on the market) Nitrification in managed systems can result in nitrate leaching and groundwater contamination
What is the fate of NO3- following nitrification? plant uptake } biological uptake (assimilatory nitrate reduction) microbial uptake accumulation (disturbed vs. managed) fixation within clay minerals leaching (groundwater contamination) • dissimilatory nitrate reduction • nitrate ammonification • denitrification Assimilatory nitrate reduction many plants prefer nitrate which is reduced in the plant prior to use however, nitrogen in fertilizer is added as ammonia or urea. microorganisms prefer ammonia since uptake of nitrate requires a reduction step assimilatory nitrate reduction is inhibited by ammonium nitrate is more mobile than ammonium leading to leaching loss
Dissimilatory reduction of nitrate to ammonia (DNRA) use of nitrate as a TEA (anaerobic process) – less energy produced inhibited by oxygen not inhibited by ammonium found in a limited number of carbon rich environments stagnant water sewage plants some sediments Denitrification use of nitrate as a TEA (anaerobic process) – more energy produced many heterotrophic bacteria are denitrifiers produces a mix of N2 and N2O inhibited by oxygen not inhibited by ammonium Dissimilatory nitrate reduction
Denitrification requires a set of 4 enzymes: nitrite reductase nitrous oxide reductase nitrate reductase nitric oxide reductase High [NO3-] favors N2 production Low [NO3-] favors N2O production
NO3 NO N2O N2 returns fixed N to atmosphere: get formation of NO, N2O Reaction of N2O with ozone O2 + UV light O + O O + O2 O3 (ozone generation) N2O + UV light N2 + O* N2O + O* 2NO (nitric oxide) NO + O3 NO2 + O2 (ozone depletion) NO2 + O* NO + O2 Denitrification NO, N2O deplete the ozone layer
Summary for nitrate reduction 1. Assimilatory nitrate reduction Nitrate assimilated must be reduced to ammonia for use. Nitrate assimilation is inhibited by ammonia Oxygen does not inhibit this process 2. Dissimilatory nitrate reduction to ammonia (DNRA) Anaerobic respiration using nitrate as TEA Inhibited by oxygen Limited to a small number of carbon-rich, TEA poor environments Fermentative bacteria predominate 3. Dissimilatory nitrate reduction (denitrification) Anaerobic respiration using nitrate as TEA Inhibited by oxygen Produces a mix of N2 and N2O Many heterotrophs denitrify
10th most abundant element average concentration = 520 ppm Sulfur Cycle oxidation states range from +6 (sulfate) to -2 (sulfide)
SO42- + ATP APS + Ppi adenosine phosphosulfate APS + ATP PAPS + ADP 3’ – phosphoadenosine – 5-phosphosulfate PAPS + 2e- SO32- + PAP SO32- + 6H+ + 6e- S2- S2- + serine cysteine + H2O 1. Assimilatory sulfate reduction The form of sulfur utilized by microbes is reduced sulfur. However, sulfide (S2-) is toxic to cells. Therefore sulfur is taken up as sulfate (SO42-), and in a complex series of reactions the sulfate is reduced to sulfide which is then immediately incorporated into the amino acid serine to form cysteine. Sulfur makes up approx. 1% of the dry weight of a cell. It is important for synthesis of proteins (cysteine and methionine) and co-enzymes. Assimilatory sulfate reduction (requires a reduction of SO42- to S2-)
marine environments algae dimethylsulfoniopropionate Dimethylsulfide (DMS) Sulfur Mineralization terrestrial environments SH – CH2 - CH - COOH + H2O OH – CH2 - CH – COOH + H2S - - NH2 NH2 cysteine serine At a C:S ratio < 200:1, sulfur mineralization is favored At a C:S ratio > 400:1, sulfur assimilation is favored
H2S and DMS are photooxidized to SO42- in the atmosphere acid rain – pH < 5.6 SO42- + water H2SO4 (sulfuric acid) fossil fuel burning releases SO2 H2SO3 (sulfurous acid) Both the H2S and the DMS generated during sulfur mineralization are volatile and therefore significant amounts are released to the atmosphere. Here they are photooxidized to sulfate. Sulfide oxidation (nonbiological) Normal biological production = 1 kg SO4/ha/yr Rural production = 10 kg SO4/ha/yr Urban production = 100 kg SO4/ha/yr
kcal/mol H2S + 1/2O2 S0 + H20 G = -50.1 Chemolithotrophic bacteria Beggiatoa Thioplaca Thiothrix Thermothrix Thiobacillus Aerobic sulfur oxidation H2S not released to the atmosphere acts as substrate for sulfur-oxidizers. Under aerobic conditions: What unusual community is based on the chemoautrophic sulfur oxiders?
What is the conundrum for these organisms? Most of these microbes deposit S0 as granules inside the cell. They can further oxidize S0 but this is not preferred. However, there are some sulfur oxidizers most notably Thiobacillus thiooxidans that are acidophilic and prefer to oxidize S0 to SO42-.
H2S + 1/2O2 S0 + H2O acid tolerant spp. S0 + 3/2O2 + H2O H2SO4 G = -149.8 kcal/mol All sulfur oxidizers are aerobic with the exception of: Acidothiobacillus denitrificans - uses nitrate as TEA 4NO3- + 3S0 3SO42- + 2N2 Acidophilic sulfur-oxidizers: Acidothiobacillus - obligate aerobes acid intolerant spp.
CO2 + H2S C(H2O) + S0 Anaerobic photosynthesis CO2 + H2O C(H2O) + O2 Aerobic photosynthesis Chromatium Ectorhodospirillum Chlorobium Under anaerobic conditions, H2S is utilized by photosynthetic bacteria: Phototrophic oxidation anaerobic photoautotrophic process: Green and purple sulfur bacteria
anaerobic heterotrophic limited number of electron donors (substrates) lactic acid pyruvic acid H2 small MW alcohols Summary - Consequences of Sulfur Oxidation • Solubilization and leaching of minerals, e.g., (phosphorus) due to decreased pH • Acid mine drainage • Acid rain Dissimilatory sulfate reduction and sulfur respiration Heterotrophic reduction of sulfur 1. respiratory S0 reduction 2. dissimilatory SO42- reduction
Desulfuromonas acetoxidans Desulfovibrio Desulfotomaculum H2 + SO42- H2S + 2H2O- + 2OH- Example of a heterotrophic sulfate reducer: CH3COOH + 2H2O + 4S0 2CO2 + 2H2S Examples of autotrophic sulfate reducers: Summary - Sulfate Reduction: • inhibited by oxygen • can result in gaseous losses to atmosphere • produces H2S which can result in anaerobic corrosion of steel and iron set in sulfate-containing soils
Winogradsky column – great illustration of sulfur cycling Set up: Soil is mixed with 1 g CaCO3, 1 g CaSO4, and shredded paper (cellulose). Soil is added to a column and saturated with water. A soil-water slurry is poured on top of this layer to the desired thickness. Column is incubated under lights or in a window.
Population development Initial conditions – aerobic, but O2 is used up quickly – aerobic chemoheterotrophs Second population – anaerobic, chemoheterotrophs ferment cellulose to low molecular weight fatty acids and alcohols Third population – anaerobic, chemoheterotrophs respire the low molecular weight fatty acids and alcohols using SO4 as the TEA. SO4 H2S (black) + CO2 Sulfate reducers Fourth population – anaerobic, photoautotrophs photosynthesize using H2S and CO2. CO2 + H2S S0 + C(H2O) Green and purple sulfur bacteria
9/12/03 9/19/03 10/2/03 9/5/03 9/26/03