Double Elimination: Making Alkynes with Two Halogens
70 likes | 166 Views
When a compound with two halogens undergoes two E2 eliminations, the result can be an alkyne if the halogens are on the same carbon. The process requires a strong base like NH2- for the second elimination. Sodium amide in liquid ammonia and sodium ethoxide in ethanol are common reagents. The choice between amide and ethoxide depends on the specific characteristics of the reaction. Examples include reactions using KOH/EtOH, NaNH2/NH3 (liq), and NaOEt/EtOH. The reaction can be conducted in two steps, often involving sodium ethoxide for the second step. When the halogens are on different carbons, a diene is typically produced in the reaction.

Double Elimination: Making Alkynes with Two Halogens
E N D
Presentation Transcript
MAKING ALKYNES “DOUBLE ELIMINATION”
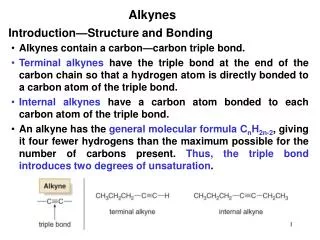
COMPOUNDS WITH TWO HALOGENS If you have a compound with two halogens it can react twice (two E2 eliminations). If both halogens are on the same carbon, an alkyne is produced. The second elimination is more difficult than the first one - it requires a stronger base. more difficult, requires a stronger base like NH2- most E2 bases will work
AMIDE VS. ETHOXIDE .. .. : : N O CH2CH3 H .. H more basic (N is less electro- negative then O) less basic (O accommodates the charge better) The usual reagent is sodium amide in liquid ammonia (-33o C): The usual reagent is sodium ethoxide in ethanol : NaNH2 / NH3 (liq) NaOEt / EtOH Both are made by adding sodium metal to the solvent.
EXAMPLES KOH EtOH D NaNH2 NH3 (liq) NaNH2 NH3 (liq) trans (the reaction is more difficult for cis )
THE REACTION CAN BE DONE IN TWO STEPS stops here with sodium ethoxide NaOEt EtOH D NaNH2 predominantly the trans isomer NH3 (liq) (lower energy product) stronger base brings about the second step
COMPOUNDS WITH TWO HALOGENS If you have a compound with two halogens it can react twice (two E2 eliminations). If the halogens are on different carbons, a diene is usually produced. KOH EtOH D NaOEt Br2 EtOH D CCl4