Moles and Solutions
610 likes | 640 Views
Moles and Solutions. Pair Trio Quartet 6-pack Dozen Baker’s dozen Score Gross. 2 3 4 6 12 13 20 144. Counting Units. Titan Lab. Created a counting unit for convenience. This is a similar process to what we do for molecules Molecules are very small

Moles and Solutions
E N D
Presentation Transcript
Pair Trio Quartet 6-pack Dozen Baker’s dozen Score Gross 2 3 4 6 12 13 20 144 Counting Units
Titan Lab • Created a counting unit for convenience. • This is a similar process to what we do for molecules • Molecules are very small • Need a very big counting unit
Titan Lab How can we figure out how many Cheerios are in a box without counting them?
Counting Large Numbers How long would it take to count to 1 million if you counted one number each second?
Counting Large Numbers How long would it take to count to 1 billion if you counted one number each second?
Counting Unit for Molecules • The mole • Abbreviated mol • Defined in terms of two different units • Masses of atoms • Measured in amu • Masses of things that are convenient to measure • Measured in grams
Moles • Define the mole in a similar way to the titan • The mass of 1 atom in amu is equal numerically to the mass of 1 mole of atoms in grams. • Where 1 amu = 1.66054 x 10-27 kg
Moles • How many things are in one mole? • Exact same process as Titan lab
Moles • 1 mol is 6.022 x 1023 items. • 1 mol of doughnuts is? • 6.022 x 1023 doughnuts • 1 mol of quizzes? • 6.022 x 1023 quizzes • 1 mol of dollar bills? • 6.022 x 1023 dollar bills
Moles are Huge Counting Units • How many years would it take to spend 1mol of dollar bills? • Assume you could spend $1 million every second of your life
Moles are Huge Counting Units • Could 1 mol of basketballs cover the Earth? • With a depth of 50 miles • How big would 1 mol of grapefruits be? • About the size of the Earth • How many human cells are on Earth? • About 1 mole of human cells • How many moles of sand grains are in the Sahara desert? • About 2 moles of sand grains
Moles Are Huge Counting Units • What is the volume of 1 mol of water molecules? • 18.01mL • Moles are huge counting units because molecules are so tiny.
How do we count moles? • Remember how we determined the number of things in a mole… • Consider carbon • The average mass of 1 carbon atom is? • 12.01amu • The mass of 1 mole of carbon atoms is? • 12.01g
Conversion Factors • Molar mass – the mass of 1 mole of atoms in grams • Found on periodic table • Numbers on periodic table are • The average mass of 1 atom in amu • The mass of 1 mole of atoms in grams • Mass on periodic table in grams = 1 mol • Avogadro’s number – 6.022x1023 things • Found on reference table • 1 mol = 6.022x1023 things
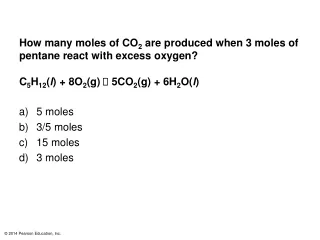
Practice Problems • How many carbon atoms are in 5.10 mol of carbon? • What is the mass of 5.10 mol of carbon?
Practice Problems • What is the mass of 2.50mol of water? • How many water molecules are in 2.50mol of water?
Practice Problems • What is the mass of 1.89x1024 molecules of ammonia? • How many formula units of calcium chloride are in 18.5g of calcium chloride?
Solutions • Solution – a homogeneous mixture of two or more compounds. • Homogeneous – looks the same throughout, mixed at the molecular level • Solvent – the compound that is the majority of a solution, the compound that is doing the dissolving • Solute – the compound that there is less of in a solution, what got dissolved in the solvent. • Can be more than one solute in a solution • Can only be one solvent
Different Solutions • Solid Dissolved in a Liquid • Salt Water • Liquid Dissolved in a Liquid • Rubbing Alcohol (isopropanol in water) • Gas Dissolved in a Liquid • An unopened Pepsi • Gas Dissolved in a Gas • Air • Solid Dissolved in a Solid • Alloys
Concentration • Need to be able to tell how much compound is dissolved in a solution. • Molarity – M – moles of solute per liter of solution • In equations, a molarity of a specific substance maybe represented using square brackets • Ex. [NH3] = 0.100M
Practice Problems • What is the molarity of a solution made by dissolving 0.125mol of sodium hydroxide in enough water to make 500.0mL of solution?
Practice Problems • What volume of 18.0M sulfuric acid is required to obtain 0.0500mol sulfuric acid?
Practice Problems • How many moles of iron(II) sulfate are in 125mL of a [FeSO4] = 0.0530M solution?
Practice Problems • 15.5g of barium chloride is dissolved in enough water to make 150mL of solution. What is the concentration of barium chloride?
Practice Problems • What volume in L of a 0.100M ammonia solution contains 4.95x1023 ammonia molecules?
Practice Problems • What mass of glucose is dissolved in 2.00L of a [C6H12O6]=0.05M solution?
Practice Problems • What volume in L of a 0.100M ammonia solution contains 4.95x1023 ammonia molecules? • What mass of glucose is dissolved in 2.00L of a [C6H12O6]=0.05M solution?
Practice Problems • What volume of a 0.150M solution of aluminum nitrate is required to obtain 5.00g of aluminum nitrate? • How many formula units of titanium(IV) oxide are in 10.0g of titanium(IV) oxide?
Electrolytes • What type of compounds are electrolytes? • Why are ionic compounds electrolytes? • Consider sodium chloride: • Is this an electrolyte? • Why? • Consider sucrose (sugar, C12H22O11): • Is this an electrolyte? • Why? • Consider calcium carbonate: • Is this an electrolyte? • Why?
Electrolytes • Why is calcium carbonate NOT an electrolyte? • Not all ionic compounds are soluble in water. • In some compounds the attractions between the ions is too great to allow them to be separated.
Solubility Rules • Located in your reference table • A compound that is soluble will dissolve in water • A compound that is insoluble will “not” dissolve in water • Solubility is really a sliding scale • Notice the solubility rules have a cutoff of 0.1M • Anything that can produce a solution of 0.1M or greater is soluble • Anything that cannot produce a solution of 0.1M is “insoluble.”
Practice Are the following soluble or insoluble in water? • Sodium iodide • Iron(III) carbonate • Silver sulfate • Calcium sulfide • Potassium phosphate
Solubility Curve • Remember everything has a different solubility in water. • Some things are hardly soluble at all • “Insoluble” • Some things are much more soluble • This is shown with a solubility curve • Shows the maximum amount of solute that can dissolve
Trends in Solubility • Most solids are more soluble as temperature increases • Most gasses are more soluble as temperature decreases. • Gas solubility can be increased by adding pressure to a liquid.
How do we describe solutions? • Concentrated – has lots of solute dissolved in it • Dilute – doesn’t have a lot of solute dissolved in it.
How do we describe solutions? • Saturated solution – a solution that can NOT dissolve more solute. • At capacity • Unsaturated solution – a solution that can dissolve more solute. • Not yet at capacity
What the heck is “Supersaturated?” • Supersaturated solution – when a solution has more solute dissolved than it should • Over capacity • NOT stable • Nucleation site – some imperfection that allows the excess solute in a solution to leave the solution • Mentos in Diet Coke • http://www.eepybird.com/
How is a supersaturated solution made? • Initially make a traditional saturated or unsaturated solution • Change the conditions on the solution so that it is then supersaturated. • Dissolve lots of solute in hot water and then cool the hot water. • Dissolve carbon dioxide at high pressure and then release the pressure.
A Real World Problem • I need 500.0mL of 1.0M hydrochloric acid. • I only have 12.0M hydrochloric acid… • How do I make the solution that I need? • Take some of the 12.0M HCl and dilute it out with water.
How much do I dilute? • How many moles of HCl need to be dissolved in the dilute solution? • Where are these moles of HCl going to come from?
How much do I dilute? • What volume of concentrated solution do I need to get that number of moles?
Practice Problems • What volume of 12.0M HCl is needed to make 500.0mL of a 1.0M HCl solution?
Practice Problems • I have roughly 750mL of 12.0M HCl left for the year. How much 6.0M HCl can I make?
Practice Problems • What is the concentration of a solution prepared by mixing 10.0mL of 18.0M H2SO4 in enough water to make 0.5000L of solution?