Acids & Bases
140 likes | 286 Views
This guide covers essential properties and naming conventions of acids and bases. Acids typically taste sour, feel itchy on the skin, and turn blue litmus paper red. Examples include vinegar and citrus fruits. Bases taste bitter, feel slippery, and turn red litmus paper blue, such as soap and milk of magnesia. Learn how to name binary and ternary acids, including common examples and naming rules based on their composition, as well as the proper way to name bases by identifying the metal and adding hydroxide.

Acids & Bases
E N D
Presentation Transcript
Acids & Bases Their Properties Naming Guidelines
Properties of Acids • Taste sour • Itchy on skin, will burn if concentrated • Turns Blue litmus paper Red • Red litmus paper stays Red Neutralization: Acid + Base Salt + Water Ex: Vinegar, Citrus Fruit
Properties of Bases • Taste Bitter • Feel Slippery • Turns Red litmus paper Blue • Blue litmus paper stays Blue • Reacts with Acid in same neutralization Ex: Soap, Milk of Magnesia, Most Cleaners
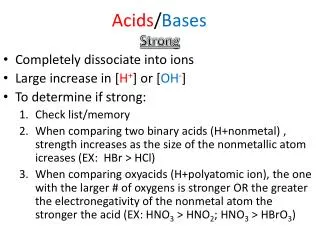
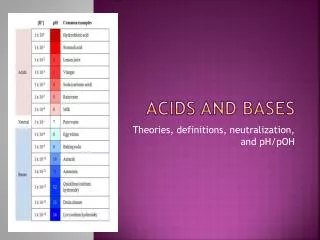
Naming Acids Binary Acids: • Contain “H” and one other element To Name: Hydro + second element name + ic + Acid Ex: HCl = Hydro chlorine – ine + ic Acid Hydrochloric Acid HF = Hydroflouric Acid
Naming Acids (cont.) Ternary Acids: • Contain “H”, “O” and one more element To Name: 3rd element name + ic + Acid Ex: HNO3 =Nitrogen – ogen + ic + Acid Nitric Acid HClO3 = Chloric Acid
Naming Acids (cont.) • If the Acid has one more Oxygen than the base acid, the naming goes as followed: To Name: Per + 3rd element + ic + Acid Ex: HNO4 = Per + Nitrogen – ogen + ic + Acid Pernitric Acid H2SO5 = Persulfuric Acid
Naming Acids (cont.) • If the Acid has one less Oxygen than the base acid, the naming goes as followed: To Name: 3rd element + ous + Acid Ex: HNO2 = Nitrogen – ogen + ous + Acid Nitrous Acid H2SO3 = Sulfurous Acid
Naming Acids (cont.) • If the Acid has two less Oxygens than the base acid, the naming goes as followed: To Name: Hypo +3rd element + ous + Acid Ex: HClO = Hypo +chlorine –ine + ous + Acid Hypochlorous Acid HNO = Hyponitrous Acid
Naming Bases • Name the metal first • Add Hydroxide Ex: NaOH = Sodium Hydroxide KOH = Potassium Hydroxide